Regulatory Science
 Research that will expand the scientific basis to inform the regulation of nicotine and tobacco products at the local, state and national levels
Research that will expand the scientific basis to inform the regulation of nicotine and tobacco products at the local, state and national levels
As a result of the Family Smoking Prevention and Tobacco Control Act of 2009, the U.S. Food and Drug Administration (FDA) now has authority to oversee and regulate tobacco products. At the same time and in response to regulatory and market pressures, the tobacco industry has intensified the development and marketing of a host of new products. Harm reduction claims, often made by health professionals, are increasingly associated with some of these.
Yet little research has been done to determine the long-term health effects and addiction potential of inhaled vaporized nicotine or the ingestion of orally delivered nicotine. The FDA’s responsibility to protect the health of the public provides an unprecedented role for the government and multiple research opportunities for the scientific community.
The FDA’s scientific framework for regulation of tobacco products includes studies on:
-
Toxicity: constituents, formulation and product design including in vitro, in vivo and human laboratory and clinical trial analyses
-
Pharmacological addiction potential
-
Abuse liability, i.e., use intensity and factors affecting use intensity in humans including product appeal, consumer perception, marketing and social influences;
-
After-market prevalence of use and health outcomes
-
Price and availability
E-cigarettes have emerged as a highly controversial new product.
Read more: Electronic Cigarettes: Are They Safe?
|


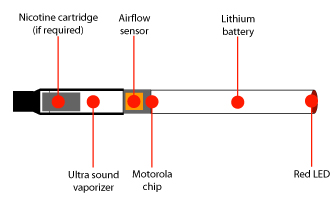 There is a lack of information on e-cigarettes regarding safety, abuse liability, and efficacy as aids to smoking cessation.
There is a lack of information on e-cigarettes regarding safety, abuse liability, and efficacy as aids to smoking cessation.